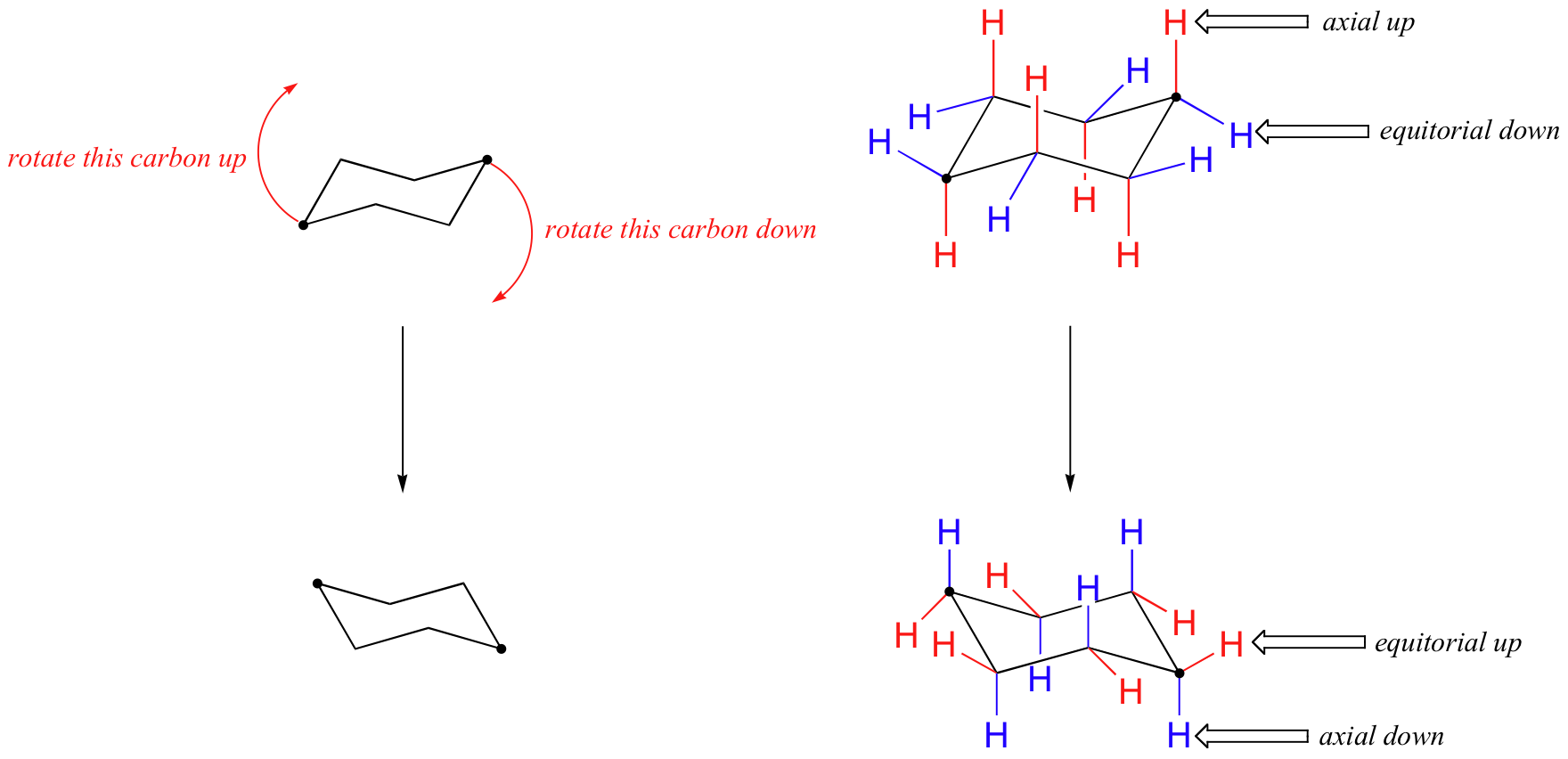
The axial bonds are vertical and alternate above and below the ring. By attaching two electrodes on a flexible molecule cyclohexane we accomplish the distinguishment of the two chair isomers of cyclohexane at room temperature using a single-molecule approach. chair conformational isomers.
Chair Conformational Isomers, Chiral centers - absolute stereochemistry sp3 carbons or nitrogens or chiral-handed left or right C A C D B behind plane forward of the plane 2. Practice doing so until you can draw it without having to refer to the figure. By attaching two electrodes on a flexible molecule cyclohexane we accomplish the distinguishment of the two chair isomers of cyclohexane at room temperature using a single-molecule approach.
 3 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts From chem.libretexts.org
3 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts From chem.libretexts.org
The axial bonds are vertical and alternate above and below the ring. Try rotating the model to look along the C-C to see the two extreme forms. Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature.
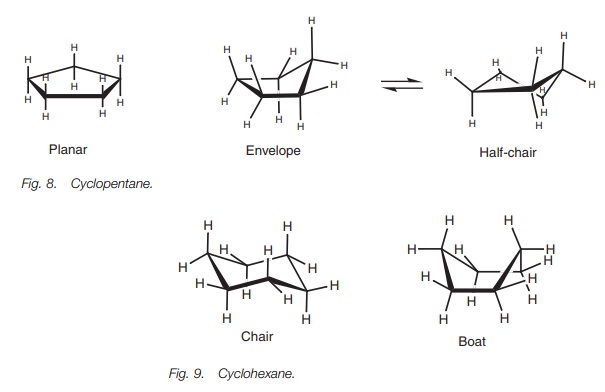
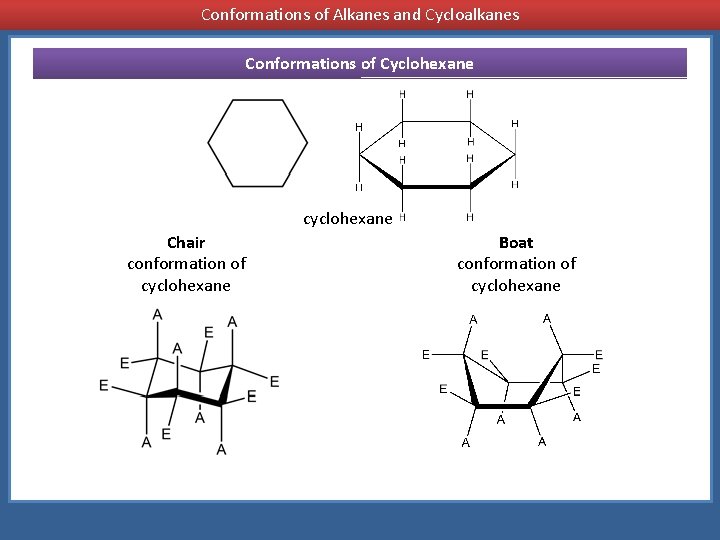
The most important shapes are chair half-chair boat and.
Anti left and syn center. Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. The most important shapes are chair half-chair boat and. In the other compound both chair structures have comparable energies so both will be populated significantly. Practice doing so until you can draw it without having to refer to the figure. Examine the model from different points of view and note the different types of strain.
Another Article :
And we learned that for a given cyclohexane the axial conformer is less stable than the corresponding equatorial conformer. In the all-equatorial isomer the carbon chlorine bond dipole moments reinforce one another leading to a large molecular moment. By attaching two electrodes on a flexible molecule cyclohexane we accomplish the distinguishment of the two chair isomers of cyclohexane at room temperature using a single-molecule approach. The chair conformation is the most stable conformer. And we learned that for a given cyclohexane the axial conformer is less stable than the corresponding equatorial conformer. Illustrated Glossary Of Organic Chemistry Isomer.
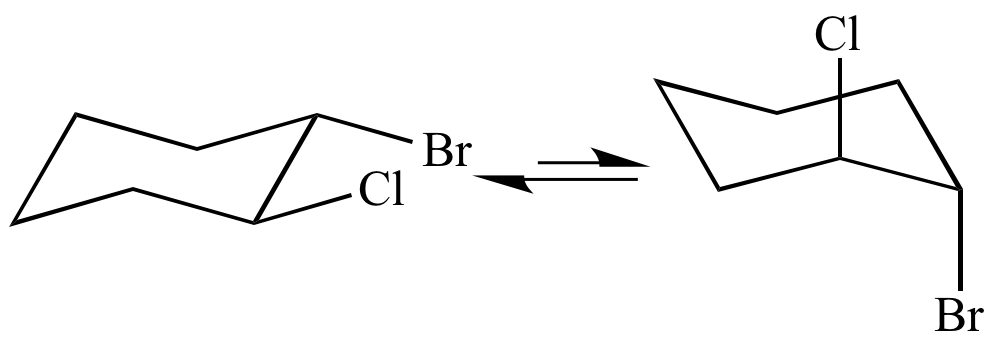
Practice doing so until you can draw it without having to refer to the figure. 33 Conformational Isomers 49 11 Kcal 55 Kcal 16 Kcal chair half-chair twist boat When the cyclohexane ring contains substituents the chair forms that result from the conformational flipping can be of different energy. Configurational isomers - separable isomers that do not readily interconvert 1. This conformation is called the chair conformation. The conformational isomerism is very understandable if it is remembered that axial and equatorial valences exchange upon chair chair interconversion. Conformational Isomers.
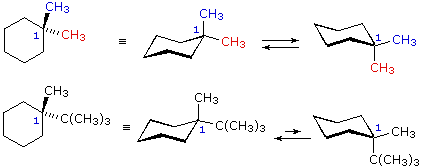
The most important shapes are chair half-chair boat and. Stereo view of chair conformation of 1 To animate see Table 3 All low temperatures NMR shifts and coupling data accord with a chair conformation and isomerism involves interchange between two such forms a process that interchanges protons between equatorial and axial environments. In the previous two posts we have talked about drawing the ring-flip of chair conformations and the A value 13-diaxial interactions. Anti left and syn center. Rotation about the C2-C3 σ bond is animated right. 3 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts.

And we learned that for a given cyclohexane the axial conformer is less stable than the corresponding equatorial conformer. Rotation about the C2-C3 σ bond is animated right. Practice doing so until you can draw it without having to refer to the figure. The different conformations are called conformers a blend of the words conformation and isomer. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. 3 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts.
Practice doing so until you can draw it without having to refer to the figure. Rotation about the C2-C3 σ bond is animated right. The conformational isomerism is very understandable if it is remembered that axial and equatorial valences exchange upon chair chair interconversion. B Characterizing cyclohexane cySMe by NMR at room temperature leads to a dynamic averaging signal. This conformation is called the chair conformation. 4 7 Cyclohexane Conformations Chemistry Libretexts.
The conformational isomerism is very understandable if it is remembered that axial and equatorial valences exchange upon chair chair interconversion. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. 33 Conformational Isomers 49 11 Kcal 55 Kcal 16 Kcal chair half-chair twist boat When the cyclohexane ring contains substituents the chair forms that result from the conformational flipping can be of different energy. This conformation is called the chair conformation. 416 Use a molecular modeling kit to build the following half-chair conformation. Chem 351 Fall 2004 Mt Thermodynamics.
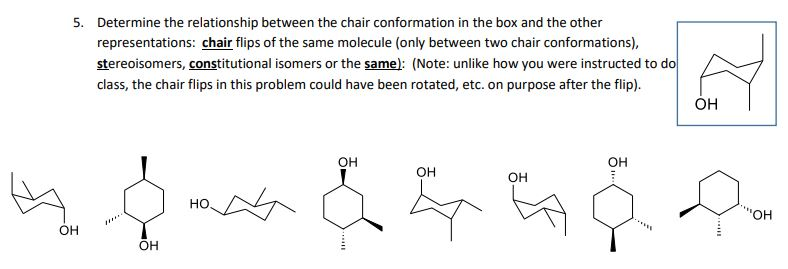
Each carbon has an axial bond and an equatorial bond. The different conformations are called conformers a blend of the words conformation and isomer. Low temperature conformations and AM1 studies. Anti left and syn center. Try rotating the model to look along the C-C to see the two extreme forms. Solved 5 Determine The Relationship Between The Chair Chegg Com.

Chiral centers - absolute stereochemistry sp3 carbons or nitrogens or chiral-handed left or right C A C D B behind plane forward of the plane 2. Cyclohexane is unique in being the only cyclic hydrocarbon which is. Enantiomers enantio opposite non-identical non-superimposable mirror image R recto right-handed. Anti left and syn center. Low temperature conformations and AM1 studies. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
Conformational Stereoisomers of Butane Butane has a larger and moreshow more content Thus the cyclohexane ring tends to assume certain non-planar warped conformations which have all angles closer to 1095 and therefore a lower strain energy than the flat hexagonal shape. In examining possible structures for substituted cyclohexanes it is useful to follow two principles. Examine the model from different points of view and note the different types of strain. By attaching two electrodes on a flexible molecule cyclohexane we accomplish the distinguishment of the two chair isomers of cyclohexane at room temperature using a single-molecule approach. Draw the chair conformation of cyclohexane in Figure 4-28 using the steps described above. Introduction To Stereochemistry Tree Of Stereochemistry Isomers Are.
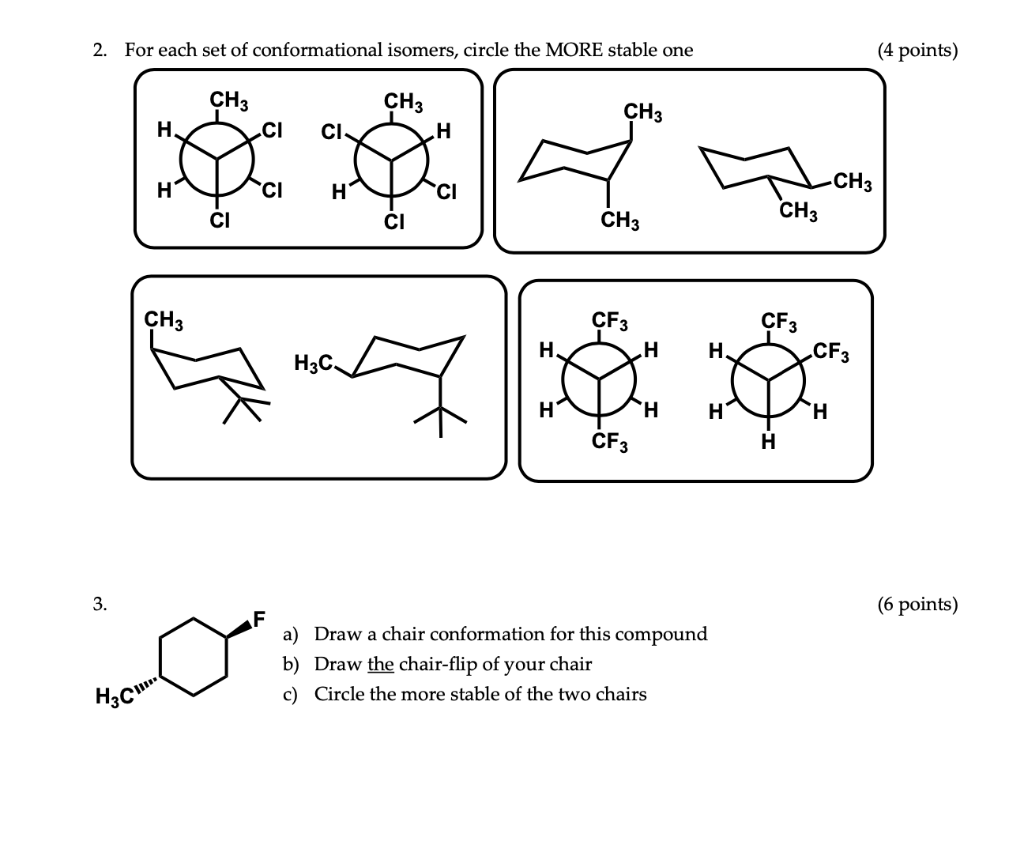
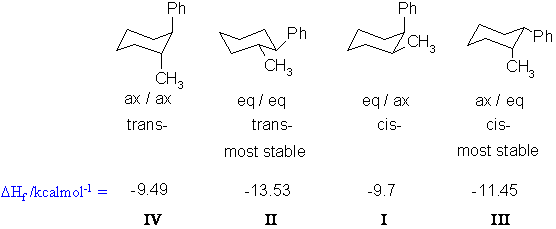
A Molecule structures of cySMe and cySAc which have two chair conformational isomers named by aa and ee respectively. The more stable conformational isomer also called a conformer is the one usually with the least crowding of substituents. In examining possible structures for substituted cyclohexanes it is useful to follow two principles. For example to draw the trans isomer of 3-methylcyclohexanol one of the groups must be equatorial and the other axial. The axial bonds are vertical and alternate above and below the ring. Solved 2 For Each Set Of Conformational Isomers Circle The Chegg Com.
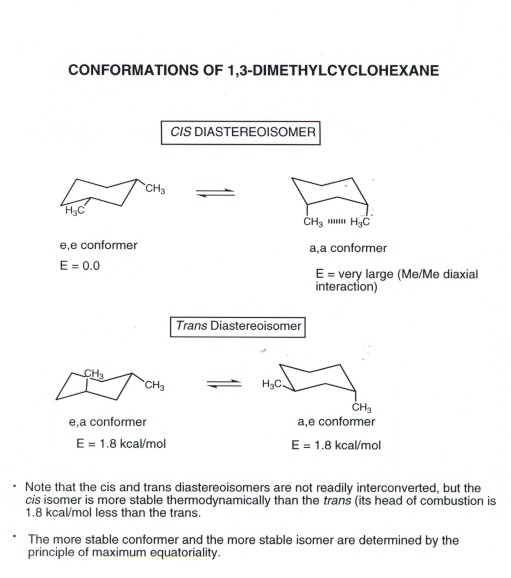
The different conformations are called conformers a blend of the words conformation and isomer. Each carbon has an axial bond and an equatorial bond. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. A Molecule structures of cySMe and cySAc which have two chair conformational isomers named by aa and ee respectively. Cyclohexane Conformational Analysis.

Rotation about the C2-C3 σ bond is animated right. Draw the chair conformation of cyclohexane in Figure 4-28 using the steps described above. Remember configurational stereoisomers are stable and do not easily interconvert whereas conformational isomers normally interconvert rapidly. Cyclohexane is unique in being the only cyclic hydrocarbon which is. For example to draw the trans isomer of 3-methylcyclohexanol one of the groups must be equatorial and the other axial. Ch 3 Conformational Isomers.
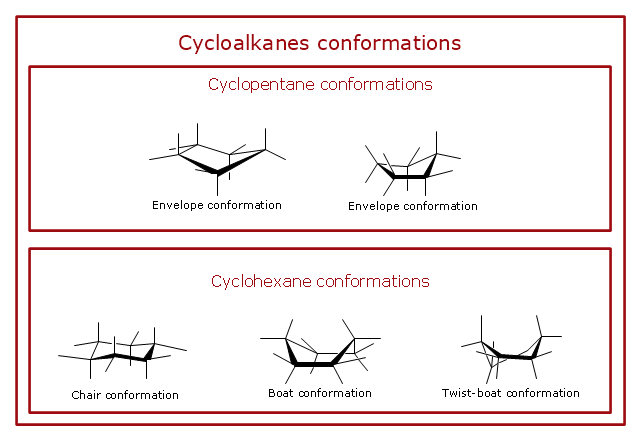
In the chair conformer of cyclohexane all the bond angles are 111 which is very close to the ideal tetrahedral bond angle of 1095 and all the adjacent bonds are staggered. Chiral centers - absolute stereochemistry sp3 carbons or nitrogens or chiral-handed left or right C A C D B behind plane forward of the plane 2. The axial bond on one of the uppermost carbons is up the. By attaching two electrodes on a flexible molecule cyclohexane we accomplish the distinguishment of the two chair isomers of cyclohexane at room temperature using a single-molecule approach. For example to draw the trans isomer of 3-methylcyclohexanol one of the groups must be equatorial and the other axial. Cycloalkanes Conformations Design Elements Conformations Conformational Isomers Of Cyclopropane.
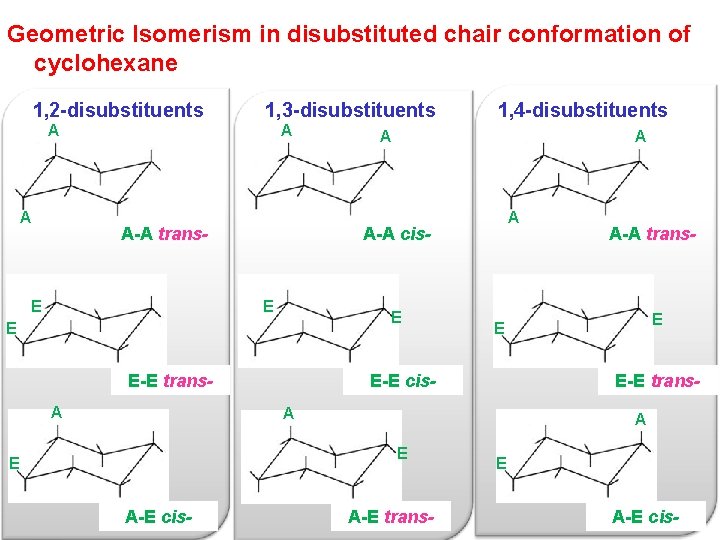
The different conformations are called conformers a blend of the words conformation and isomer. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. Examine the model from different points of view and note the different types of strain. 33 Conformational Isomers 49 11 Kcal 55 Kcal 16 Kcal chair half-chair twist boat When the cyclohexane ring contains substituents the chair forms that result from the conformational flipping can be of different energy. Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational.
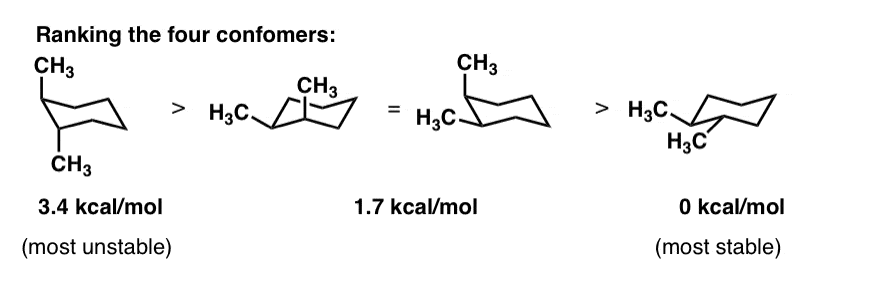
The identification of conformational isomers of the flexible molecule is challenging owing to the rapid interconversion of isomers. Practice doing so until you can draw it without having to refer to the figure. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. The more stable conformational isomer also called a conformer is the one usually with the least crowding of substituents. Chiral centers - absolute stereochemistry sp3 carbons or nitrogens or chiral-handed left or right C A C D B behind plane forward of the plane 2. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.