Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top. The chair conformation in which the methyl group is equatorial is the most stable while the alternative chair conformation in which the methyl group is axial is 73 kJmol more energetic. chair conformation geometry.
Chair Conformation Geometry, Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. However keep in mind that the geometry of the cyclohexane needs to be. An alternate conformation for a six-membered ring is called the boat.
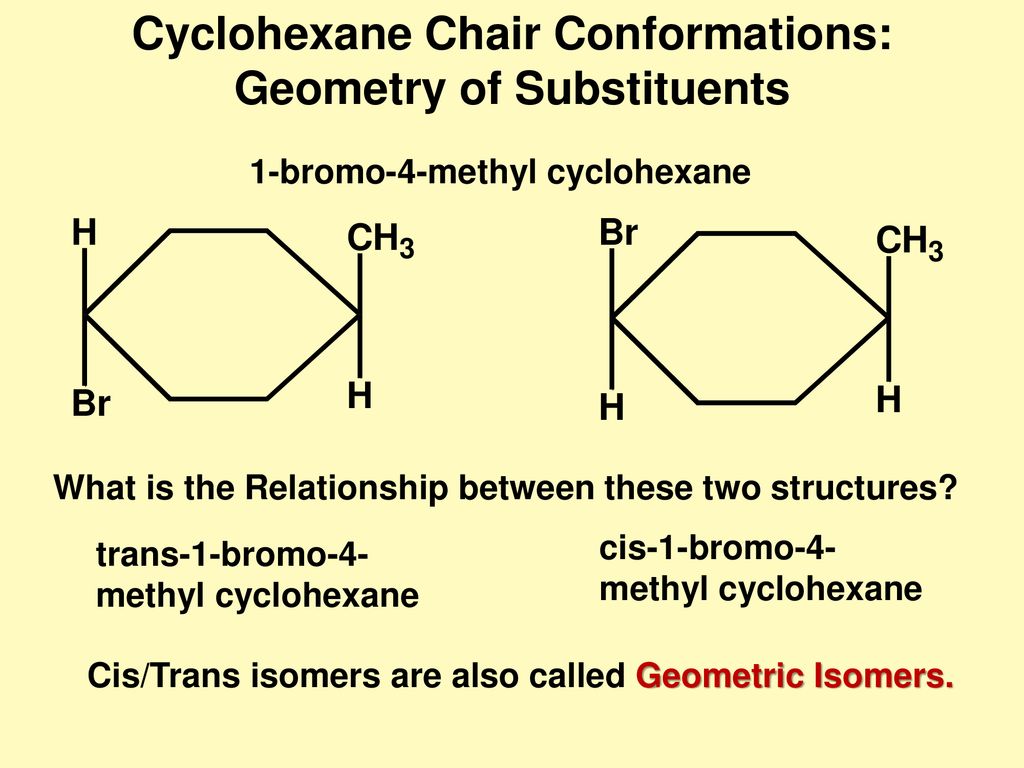 Ch 4 5 Cycloalkane Conformations I Ppt Download From slideplayer.com
Ch 4 5 Cycloalkane Conformations I Ppt Download From slideplayer.com
Geometry vibration frequencies and IR absorption intensities of 6-radialene considering the planar and chair form. The CH2 bending frequencies. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation.
Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion.
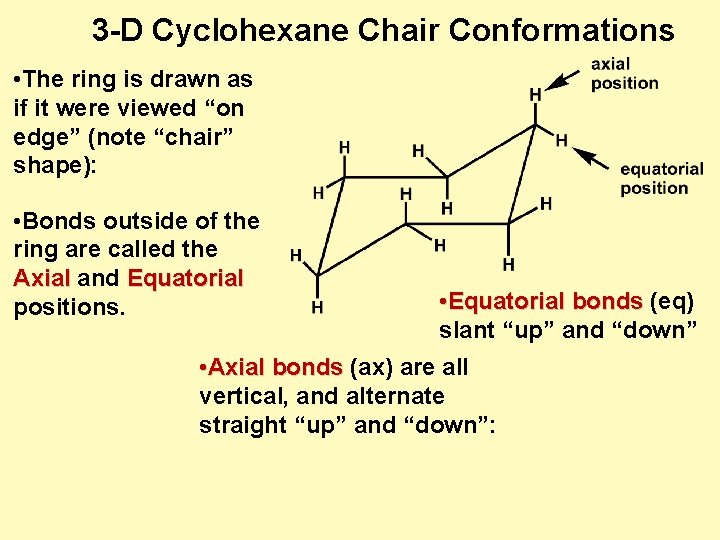
NB that your cyclohexane might not initially be in this conformation if you are very unlucky and have somehow managed to arrange the atoms in such a manner that they are closer to one of the other conformations than the chair one and in that case we will have to rearrange them to our desired conformation. The chair conformation of cyclohexane is not rigid. I cant see how the structure drawn is the enantiomer. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here. In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. To build the chair conformation.
Another Article :

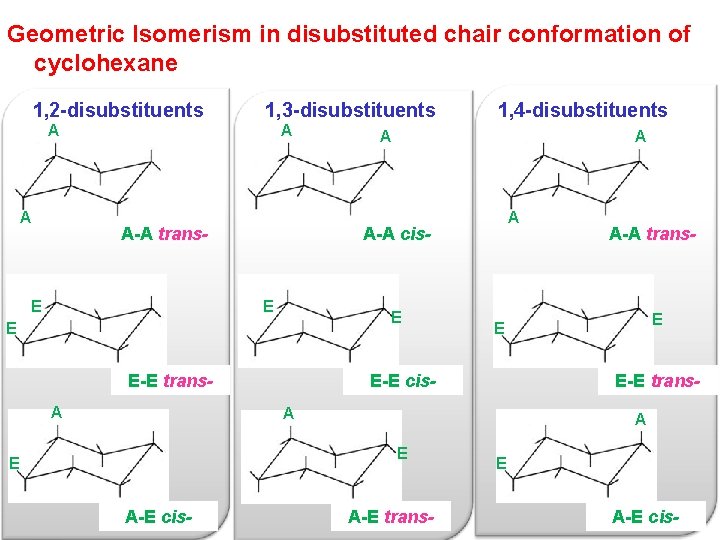
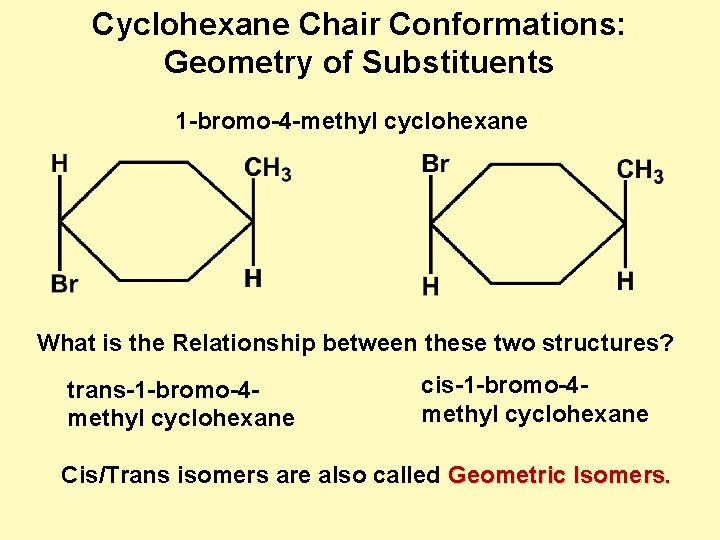
This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings. Always place the largesthighest priority group in the equatorial position. How can geometric isomerism arise due to. The chair conformation in which the methyl group is equatorial is the most stable while the alternative chair conformation in which the methyl group is axial is 73 kJmol more energetic. What is the most stable chair conformation. 4 C 1 Chair Conformation Of A Hexopyranose And Newman Projections Of Download Scientific Diagram.
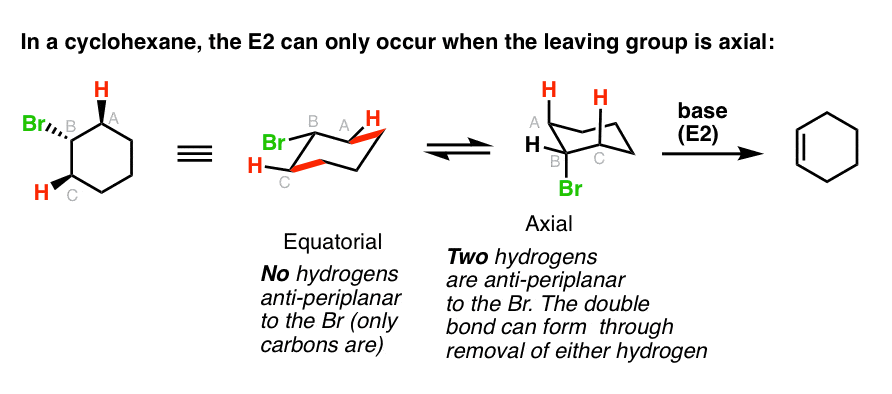
Choose Labels on the Display menu and label the atoms by number. The CC stretching frequencies of the chair form are found to be higher than those of the planar ones. Chair is the most stable conformation of cyclohexane. Playing with a model can help in learning that. In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. Antiperiplanar Relationships The E2 Reaction And Cyclohexane Rings.
The chair conformation of cyclohexane is not rigid. I cant see how the structure drawn is the enantiomer. The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle. Most commonly encountered in the chair conformation of cyclohexane. Need help preparing for the Organic Chemistry section of the MCAT. Cyclohexane Conformation Wikiwand.
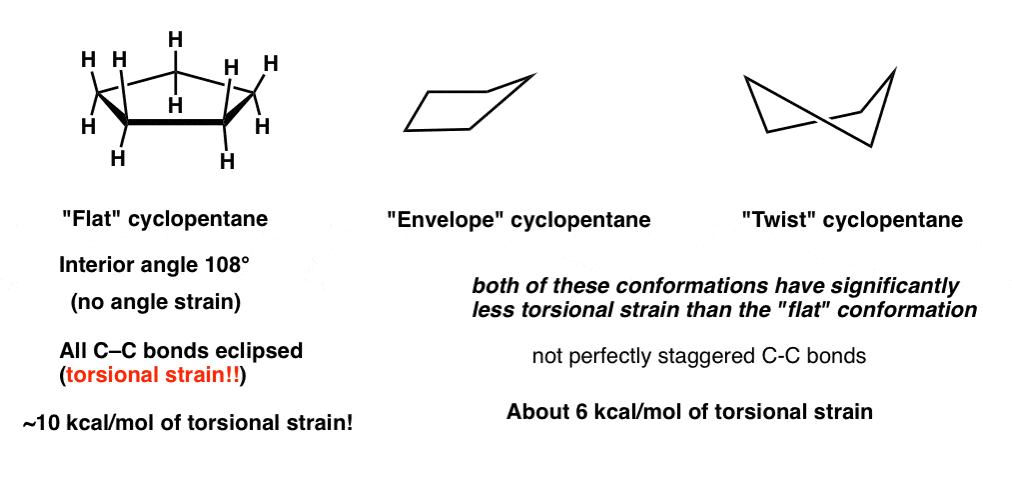
The CC stretching frequencies of the chair form are found to be higher than those of the planar ones. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. Viewed 2k times 2 begingroup I have two questions regarding this solution. This specific conformation that we are going to look at in a moment is called the chair conformation. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. Cyclohexane Conformations Master Organic Chemistry.
The chair conformation of cyclohexane is not rigid. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. All of the bond angles are close to tetrahedral but close contact between flagpole hydrogens causes van der Waals strain in boat. Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational.
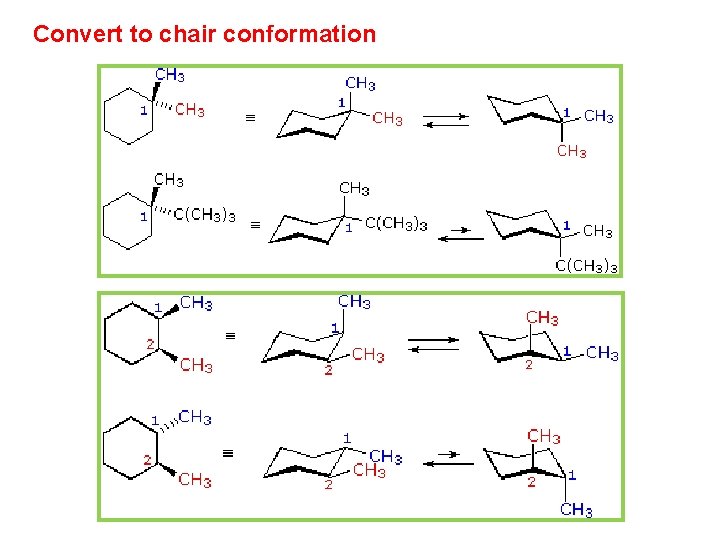
This specific conformation that we are going to look at in a moment is called the chair conformation. Playing with a model can help in learning that. So that it looks exactly like the chair conformation shown on the left in Figure 4-33b. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. It can convert to a twist boat comformation and then to a new chair conformation in a process termed ring-flipping as shown Figure 614 not all the hydrogens are shown for clarity. Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational.

Chair is the most stable conformation of cyclohexane. Building Chair Cyclohexane. It can convert to a twist boat comformation and then to a new chair conformation in a process termed ring-flipping as shown Figure 614 not all the hydrogens are shown for clarity. The Chair Conformation The stability data in Table 71 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkanevery close to the ideal 1095 tetrahedral angle. I cant see how the structure drawn is the enantiomer. Stereoisomeric Conformations Different Perspectives Achiral Stereoisomers And Meso Compounds Organic Chemistry Solutions.

What is the most stable chair conformation. The rest are considered as a twisted conformation. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. However keep in mind that the geometry of the cyclohexane needs to be. The CC stretching frequencies of the chair form are found to be higher than those of the planar ones. Plausible Configurations Adopted By The L Pipecolic Acid Molecule Download Scientific Diagram.

The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. In the ring-flipping process C. If two opposite torsions are close to 0 margin 30 and the other remaining torsion angles are around - 60 then it is considered a boat conformation. The most stable conformation for cyclohexane. To build the chair conformation. Organic Chemistry Educational Infographic Cyclohexane And Chair Conformation Video Organic Chemistry Chemistry Educational Infographic.

The CH2 bending frequencies. YOUR TURN Draw each line structure as a Haworth projection and each Haworth projection as a line structure including dashwedge notation. What is the most stable chair conformation. Set the select level to Atoms. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. Cyclohexane Conformations A Top Chair Conformation B Middle Download Scientific Diagram.
If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. The most stable conformation for cyclohexane. The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle. MedSchoolCoach expert Ken Tao will teach everything you need to know about chair conform. Playing with a model can help in learning that. Ch 4 5 Cycloalkane Conformations I Cyclohexane Conformations.
The CC stretching frequencies of the chair form are found to be higher than those of the planar ones. This geometry of chair cyclohexane conformations is generally preserved when the hydrogen atoms are replaced by halogen atoms such as fluorine chlorine bromine and iodine. Geometry vibration frequencies and IR absorption intensities of 6-radialene considering the planar and chair form. Playing with a model can help in learning that. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. Ch 4 5 Cycloalkane Conformations I Cyclohexane Conformations.
180 pm Boat conformation is less stable than the chair. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. Chair conformation chirality. Most commonly encountered in the chair conformation of cyclohexane. What Is Chair Conformation Quora.

In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. An alternate conformation for a six-membered ring is called the boat. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. Without flipping the chair simply rotate the molecule again until it looks like the chair conformation on the right. Rules Of Thumb Rots For Chair Conformations And Substituent Stability Teach The Mechanism.

The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. The most stable conformation for cyclohexane. Set the Default Element to carbon and get into drawing mode. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. How can geometric isomerism arise due to. Cyclohexane Conformation Wikiwand.











