In the previous two posts we have talked about drawing the ring-flip of chair conformations and the A value 13-diaxial interactionsAnd we learned that for a given cyclohexane the axial conformer is less stable than the corresponding equatorial conformerFor example the energy difference of the axial and equatorial isopropyl cyclohexane is 92 kJmol. Calculate the difference in Gibbs free energy between the alternative chair conformations of trans-4-iodo-1-cyclohexanol. chair conformation cis vs trans.
Chair Conformation Cis Vs Trans, 2 methylcyclohexanol is an alcohol. There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans. Cyclohexane is a cycloalkane in which a six-membered carbon ring that exists in the chair conformation.
 Identify Cis And Trans Isomers From The Fo Clutch Prep From clutchprep.com
Identify Cis And Trans Isomers From The Fo Clutch Prep From clutchprep.com
We will also discuss the relationship between cistrans and axialequatorial. There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans. Furthermore since 14-axax-bonds are always trans opposite direction.
There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans.
The most stable isomer for disubstituted cyclohexanes is summarized below. Contrary to open-chain alkenes cis cycloalkenes in general are more stable than their trans isomers. Cholesterol testosterone are trans- rigid dislike. Conformational Analysis of Disubstituted Cyclohexanes 14-dimethylcyclohexane. As a result trans-1-ethyl-2-methylcyclohexane has a more stable chair conformer than cis-1-ethyl-2-methylcyclohexane. We will also discuss the relationship between cistrans and axialequatorial.
Another Article :

Comb is 5KJmol higher for the cis isomer 69 312. The following is the correct way to draw chair cyclohexane. Like in given figure no. What I mean by face is just top or bottom. We understand that the best path the lowest energy path available. How To Identify Cis And Trans Forms Of Cyclohexane Chemistry Stack Exchange.

Which is more stable cis- or trans- isomers. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. The reason why equatorial is more stable is because youre avoiding strain thats caused by substituents repelling each other when theyre in the axial position. Chair concordances can present the challenge in organic chemistry. 2 methylcyclohexanol is an alcohol. Difference Between Cis And Trans Cyclohexane Compare The Difference Between Similar Terms.

The following is the correct way to draw chair cyclohexane. Difference Between Cis and Trans Cis-trans isomerism consists in the possibility of placing substituent groups on one or on different sides of a double bond plane or a non-aromatic cycle. The reason why equatorial is more stable is because youre avoiding strain thats caused by substituents repelling each other when theyre in the axial position. Cis and trans is actually going to be based on whether the groups are facing the same face of the ring. Can only detect less than 5000 charactersstabile cis-1-ethyl-2-methylcycloesan cis-1-ethyl-2-methylcycloesan Suggestions. How To Identify Cis And Trans Forms Of Cyclohexane Chemistry Stack Exchange.
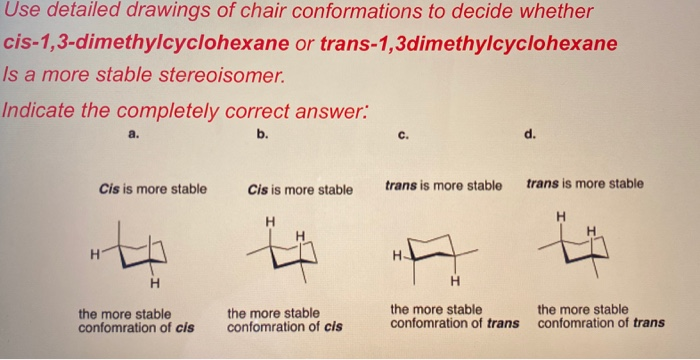
The trans double bond causes strong twisting of the ring. For cyclohexanes you may be asked to draw a chair in which case all substituents must be either axial or equatorial. Draw all the chair conformators of each isomer and decide that it is the most stable. We will look at how to show cis and trans relationships in simple hexagon structural formulas and we will look at structures showing the common chair conformation focusing on axial vs equatorial orientations. The reason why equatorial is more stable is because youre avoiding strain thats caused by substituents repelling each other when theyre in the axial position. Solved Use Detailed Drawings Of Chair Conformations To Chegg Com.

Because of the resulting high ring strain small trans cycloalkenes have not been observed and cis isomers show considerable ring strain. Chair conformation cis vs trans You should be able to quickly draw cyclohexane rings in which the axial and equatorial bonds are readily identifiable and distinguishable. We will look at how to show cis and trans relationships in simple hexagon structural formulas and we will look at structures showing the common chair conformation focusing on axial vs equatorial orientations. Trans-13-Dimethylcyclohexane cis xanewwwShimiPediaircis xane transorocyclohexane. As you can see that is what happens in the first chair. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.

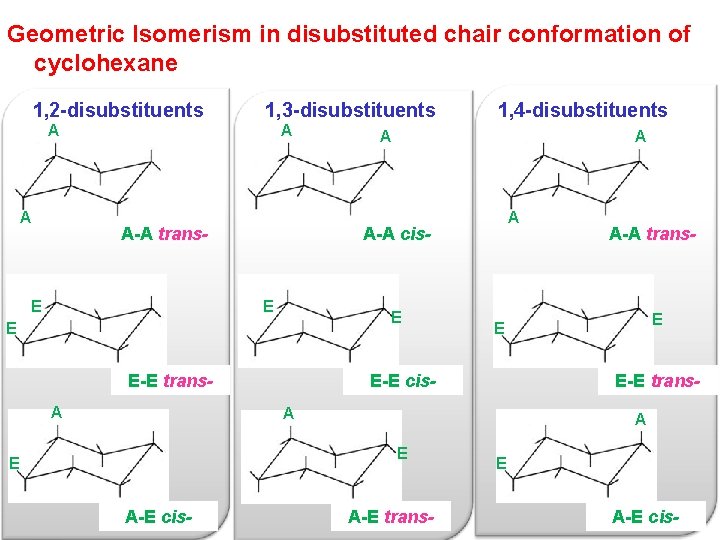
Cis trans top face bottom face a b b b b b a a a CYCLOHEXANE. Difference Between Cis and Trans Cis-trans isomerism consists in the possibility of placing substituent groups on one or on different sides of a double bond plane or a non-aromatic cycle. For cyclohexanes you may be asked to draw a chair in which case all substituents must be either axial or equatorial. 1 if both methyl groups are attached to an. But they dont have to. Organic Chemistry Stereoisomerism Of Chair Conformation Youtube.
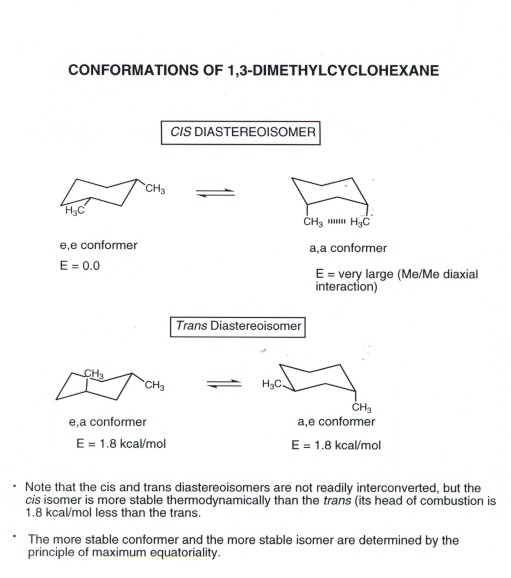
Difference Between Cis and Trans Cis-trans isomerism consists in the possibility of placing substituent groups on one or on different sides of a double bond plane or a non-aromatic cycle. Compounds with 13-axeq- or 12-eqax-bonds are always trans. Biological function of cholesterol inserts into cell membrane and stabilizes it. H 3 C CH 3 CH 3 H CH 3 H Δ kjmol. In the chair conformation this compound has the lowest. Cyclohexane Conformational Analysis.

We will also discuss the relationship between cistrans and axialequatorial. Chair concordances can present the challenge in organic chemistry. Furthermore since 14-axax-bonds are always trans opposite direction. Draw cis and trans 2 methylcyclohexanol in their most stable chair conformations. We understand that the best path the lowest energy path available. Cis And Trans Substituent Relationships Organic Chemistry I Youtube.

Cis and trans is actually going to be based on whether the groups are facing the same face of the ring. In the chair conformation this compound has the lowest. You should find that the trans isomer of 14-dimethylcyclohexane is more stable than the cis isomer. Cis- is flexible as the axial and equatorial can interconvert chair flapping process Trans- not interconvertable. Cis and trans chair conformations Warning. Determining Cis Trans On Cyclohexanes Youtube.

Cis and Trans Isomerism - YouTube. Im going to be looking at direction. Disk-like structure- rigid typical of steroids Most steroids eg. 123702 Organic Chemistry Enolate formation and geometry II Second conformation that places CH perpendicular to CO gives trans-enolate Only differs by relative position of R1 and R2 The steric interaction of R1 and R2 results in the cis-enolate normally predominating As results below demonstrate stereoselectivity is influenced by the size of R. Cholesterol testosterone are trans- rigid dislike. Possible Chair Conformations Of 1 2 Dimethylcyclohexane.
Calculate the difference in Gibbs free energy between the alternative chair conformations of trans-4-iodo-1-cyclohexanol. ΔH comb is 7 KJmol lower for the trans isomer 13-dimethylcyclohexane. Cis and trans isomers are found both among organic and inorganic compounds. Compounds with 13-axeq- or 12-eqax-bonds are always trans. Which is more stable cis- or trans- isomers. Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational.

You should find that the trans isomer of 14-dimethylcyclohexane is more stable than the cis isomer. ΔH comb is 7 KJmol lower for the cis isomer 12-dimethylcyclohexane. Because of the resulting high ring strain small trans cycloalkenes have not been observed and cis isomers show considerable ring strain. 3 kcalmol lower than cis. For cyclohexanes you may be asked to draw a chair in which case all substituents must be either axial or equatorial. Difference Between Cis And Trans Cyclohexane Compare The Difference Between Similar Terms.

Compounds with 13-axeq- or 12-eqax-bonds are always trans. Contrary to open-chain alkenes cis cycloalkenes in general are more stable than their trans isomers. To draw and identify the best cis versus trans just draw a chair with both groups equatorial and then identify whether that is cis or transStepsforDrawingtheBestNewmanprojection. The key difference between cis cyclohexane and trans cyclohexane is that cis cyclohexane has its substituents pointing to the same plane of the ring whereas trans cyclohexane has its substituents pointing to opposite planes. Compounds with 14-axax-substitutions are always trans. 4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts.

2 methylcyclohexanol is an alcohol. Biological function of cholesterol inserts into cell membrane and stabilizes it. Contrary to open-chain alkenes cis cycloalkenes in general are more stable than their trans isomers. ΔH comb is 6 KJmol lower for the trans isomer cis one equatorial one axial. Calculate the difference in Gibbs free energy between the alternative chair conformations of trans-4-iodo-1-cyclohexanol. For Cis 1 3 Dimethylcyclohexane Which Two Clutch Prep.
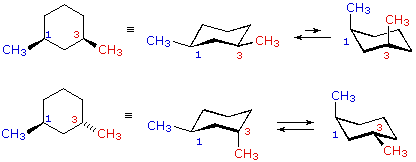
But they dont have to. To draw and identify the best cis versus trans just draw a chair with both groups equatorial and then identify whether that is cis or transStepsforDrawingtheBestNewmanprojection. Cis trans top face bottom face a b b b b b a a a CYCLOHEXANE. Cis and trans chair conformations Warning. 2 methylcyclohexanol is an alcohol. 4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts.










