There are two equivalent chair conformations. At any point on the chair that sticks down draw the axial hydrogen straight down. chair conformation axial hydrogens.
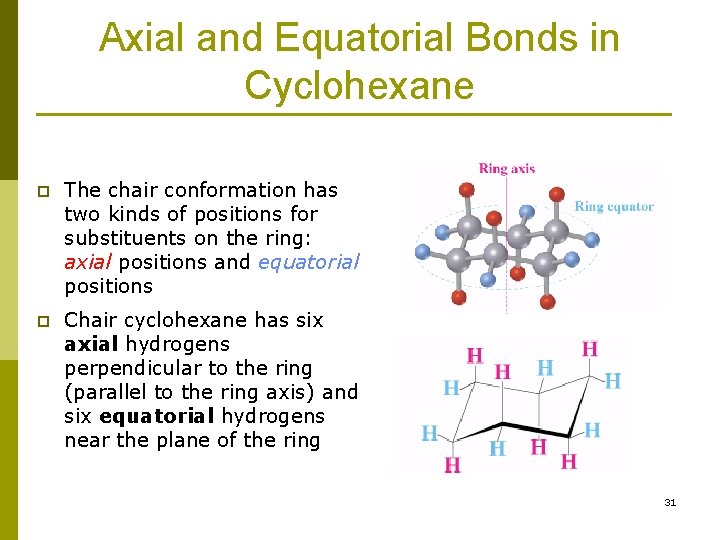
Chair Conformation Axial Hydrogens, Remember that in the chair form of cyclohexane which has already been established as the most stable conformation by several kcalmol there are hydrogens in two distinct positions. In this process the equa-torial hydrogens have become axial and the axial hydrogens have become equatorial. The bonds to one type are parallel to the axis of the ring.
 Stereochemistry Of Alkanes And Cycloalkanes The Shapes Of From slidetodoc.com
Stereochemistry Of Alkanes And Cycloalkanes The Shapes Of From slidetodoc.com
In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. These are called axial hydrogens. The total strain in the chair conformation is small and therefore the chair conformation is very stable.
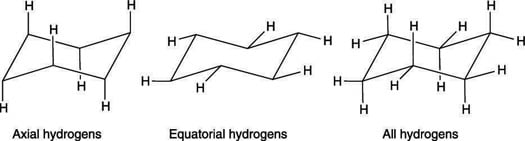
It turns out that the chair and boat conformations of cyclohexane both have 6 axial hydrogens - so the chair does not have more axial hydrogens.
The dihedral angle between two hydrogen atoms on adjacent carbon atoms on the same side of the ring is 55º. Look at the axial-methylcyclohexane in chair conformation shown. It turns out that the chair and boat conformations of cyclohexane both have 6 axial hydrogens - so the chair does not have more axial hydrogens. Of the rightmost carbon changes one chair conformation into another completely equivalent chair conformation. See also boat conformation axial bond equatorial bond A Value. The total strain in the chair conformation is small and therefore the chair conformation is very stable.
Another Article :
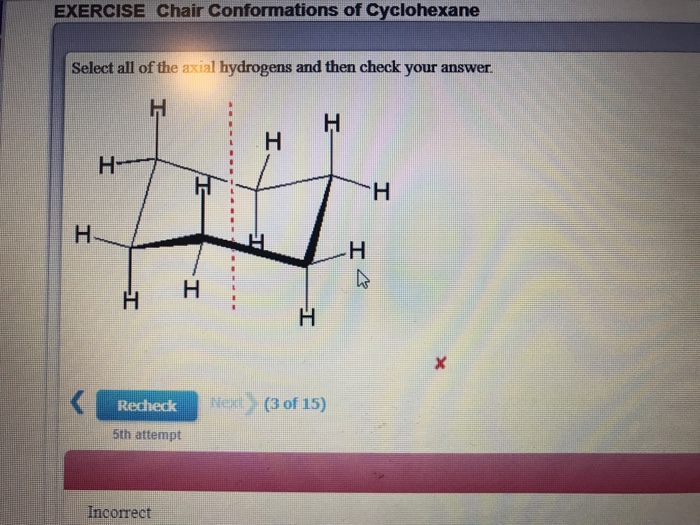
They are called equatorial hydrogens because they. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. In the chair conformation one hydrogen on each carbon is equatorial and one is axial. See also boat conformation axial bond equatorial bond A Value. Hence the angle strain in the chair conformation is very small. Solved Exercise Chair Conformations Of Cyclohexane Select Chegg Com.

The other hydrogens are directed outward from the ring. In the chair conformation one hydrogen on each carbon is equatorial and one is axial. In the chair conformation cyclohexane has two different types of hydrogens. But notice what has happened to the hydrogens. Hence the torsional strain in the chair conformation is small. Stereochemistry Of Alkanes And Cycloalkanes The Shapes Of.
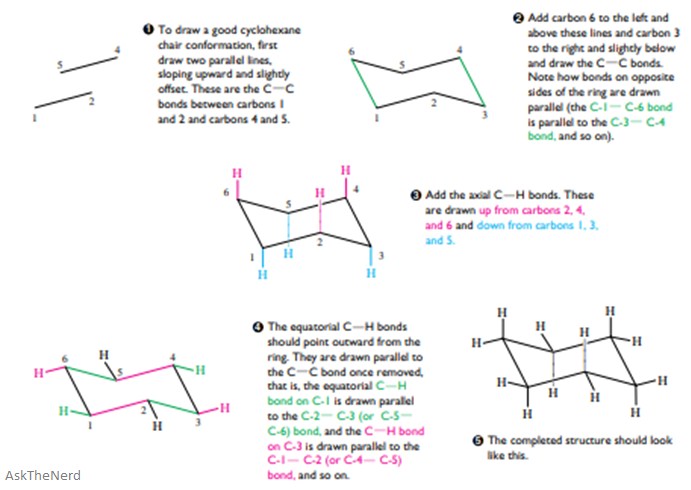
The axial bonds alternate up and down around the ring. The other hydrogens are directed outward from the ring. At any point on the chair that sticks up put the axial hydrogen sticking straight up. The total strain in the chair conformation is small and therefore the chair conformation is very stable. Axial and Equatorial Hydrogens. How To Draw The Chair Conformation Of Cyclohexane Dummies.

The hydrogens which radiate out from around the ring are called equatorial hydrogens. Give the IUPAC name for the cycloalkane shown below 1 point CH CHCH 7. At any point on the chair that sticks up put the axial hydrogen sticking straight up. Look at the axial-methylcyclohexane in chair conformation shown. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Axial And Equatorial Planes On The Chair Conformation Of Cyclohexane Organic Chemistry I Youtube.

The symmetry is D 3d. The hydrogens which radiate out from around the ring are called equatorial hydrogens. In the chair conformation of cyclohexane three hydrogens point up and three point down. Draw the two chair conformations of cis-13-dimethylcyclohexane and indicate which chair conformation is stable. 1 1 4 4 In the illustration above the two chair conformations are in equilibrium. Chapter 4 Flashcards Quizlet.

Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator. Axial and Equatorial Hydrogens In the chair conformation of cyclohexane three hydrogens point up and three point down. The symmetry is D 3d. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator. Boat Conformation Axial Hydrogens Chemistry Stack Exchange.

In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon. Draw the two chair conformations of cis-13-dimethylcyclohexane and indicate which chair conformation is stable. The dihedral angle between two hydrogen atoms on adjacent carbon atoms on the same side of the ring is 55º. These H atoms are respectively referred to as axial. The chair conformation is the most stable conformer. Axial And Equatiorial Bonds In Cyclohexane Mcc Organic Chemistry.
This is true for 1R-33-dichlorocyclohexanol. These H atoms are respectively referred to as axial. At any point on the chair that sticks up put the axial hydrogen sticking straight up. 1 1 4 4 In the illustration above the two chair conformations are in equilibrium. The axial bonds alternate up and down around the ring. Axial And Equatorial Facts Summary Definition Chemistry Revision.
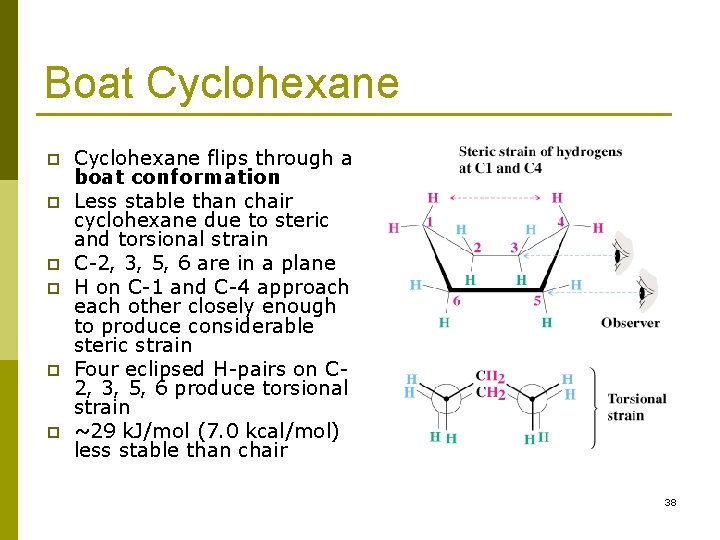
The bonds to one type are parallel to the axis of the ring. At any point on the chair that sticks up put the axial hydrogen sticking straight up. The other hydrogens are directed outward from the ring. The total strain in the chair conformation is small and therefore the chair conformation is very stable. The axial bonds alternate up and down around the ring. Stereochemistry Of Alkanes And Cycloalkanes The Shapes Of.
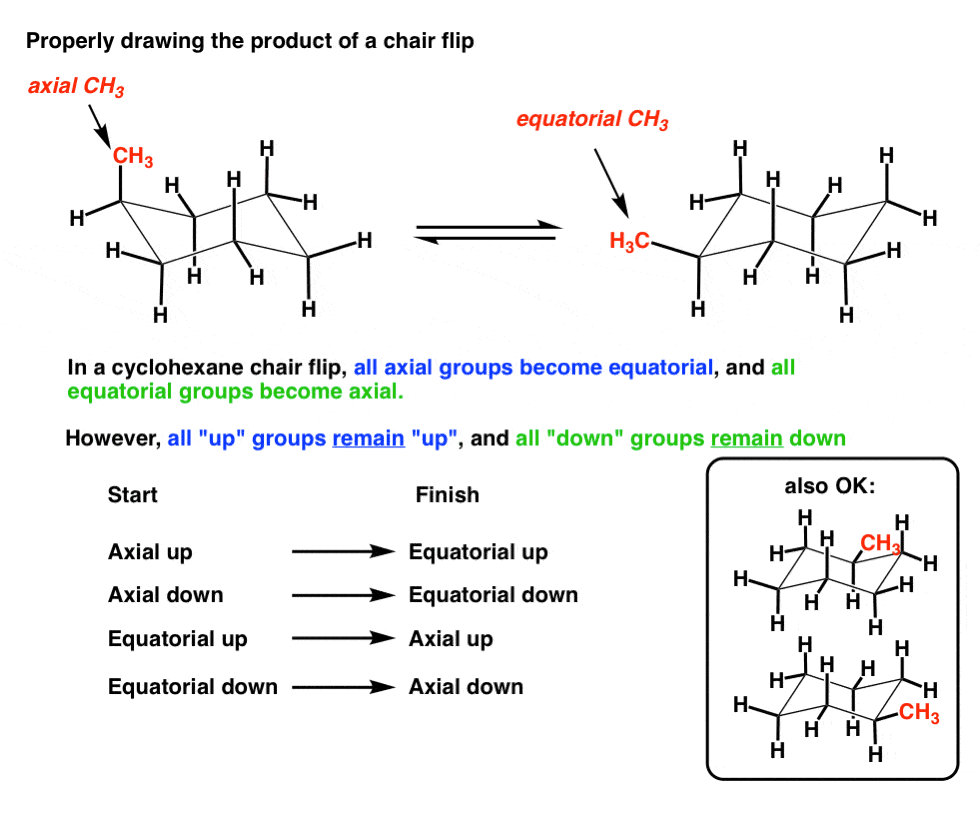
It turns out that the chair and boat conformations of cyclohexane both have 6 axial hydrogens - so the chair does not have more axial hydrogens. The hydrogens which radiate out from around the ring are called equatorial hydrogens. Each face has alternating axial and equatorial -Hs. These six hydrogens which point along an axis through the molecule are called axial hydrogens. The resulting interaction is called a. The Cyclohexane Chair Flip Master Organic Chemistry.
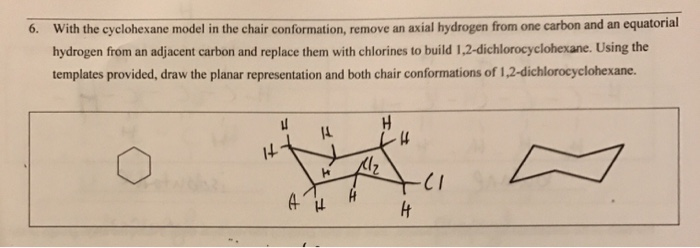
The chair conformation is the most stable conformer. Axial and Equatorial Hydrogens In the chair conformation of cyclohexane three hydrogens point up and three point down. Give the IUPAC name for the cycloalkane shown below 1 point CH CHCH 7. The symmetry is D 3d. The dihedral angle between two hydrogen atoms on adjacent carbon atoms on the same side of the ring is 55º. Solved 6 With The Cyclohexane Model In The Chair Chegg Com.
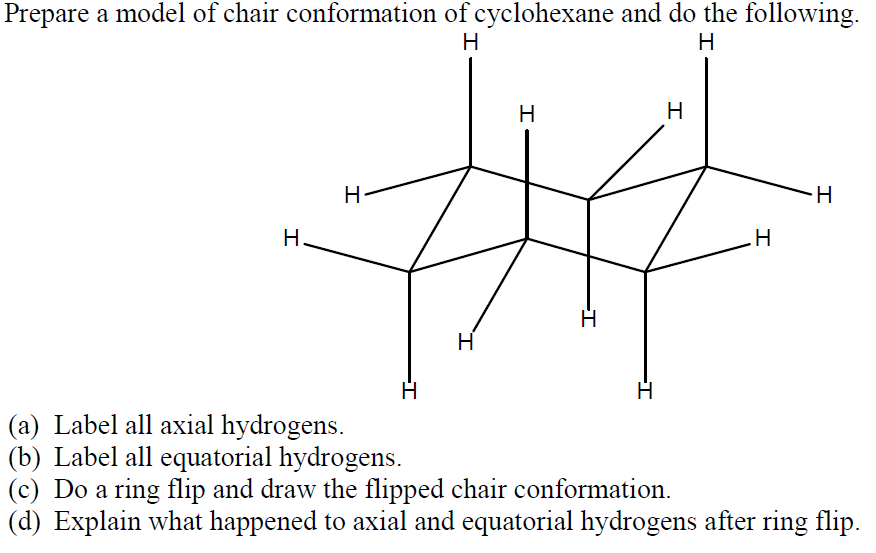
The axial bonds alternate up and down around the ring. The dihedral angle between two hydrogen atoms on adjacent carbon atoms on the same side of the ring is 55º. Look at the axial-methylcyclohexane in chair conformation shown. Hence the angle strain in the chair conformation is very small. At any point on the chair that sticks up put the axial hydrogen sticking straight up. Solved Prepare A Model Of Chair Conformation Of Cyclohexane Chegg Com.
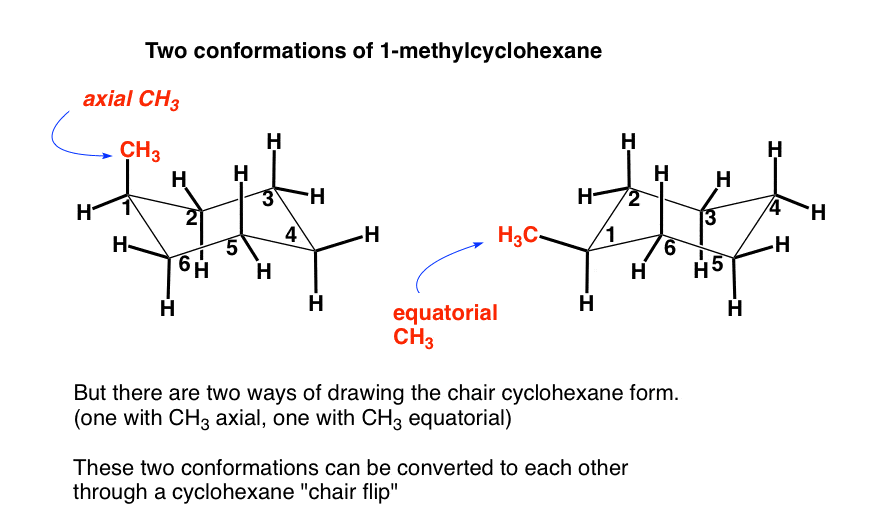
In the chair conformation of cyclohexane three hydrogens point up and three point down. In the chair conformation cyclohexane has two different types of hydrogens. The other hydrogens are directed outward from the ring. The total strain in the chair conformation is small and therefore the chair conformation is very stable. Cyclopropane is least stable. The Cyclohexane Chair Flip Master Organic Chemistry.

Each face has alternating axial and equatorial -Hs. The total strain in the chair conformation is small and therefore the chair conformation is very stable. In the chair conformation cyclohexane has two different types of hydrogens. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. These are called axial hydrogens. The Number Of Axial Hydrogen Atoms In Chair Form Of Cyclohexane Is.
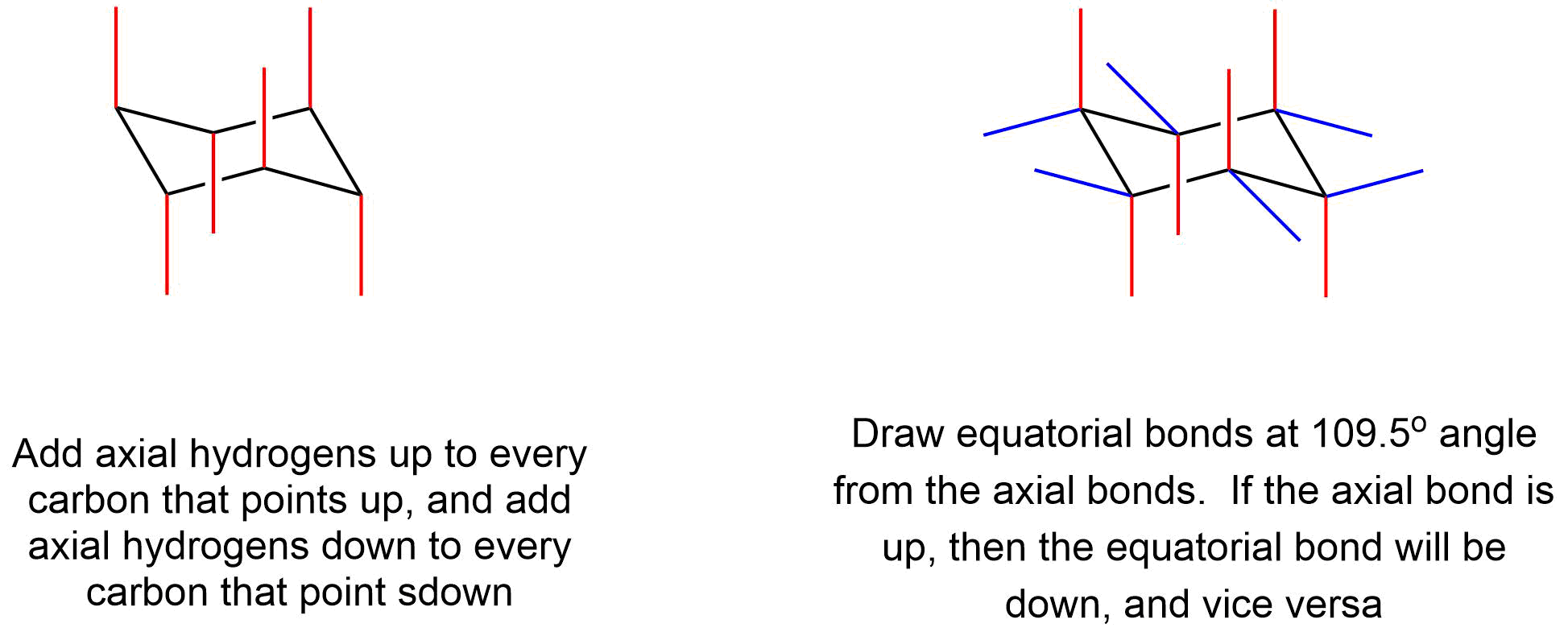
Axial and Equatorial Hydrogens In the chair conformation of cyclohexane three hydrogens point up and three point down. This is true for 1R-33-dichlorocyclohexanol. The axial bonds alternate up and down around the ring. The other hydrogens are directed outward from the ring. Of the rightmost carbon changes one chair conformation into another completely equivalent chair conformation. 3 7 Axial And Equatorial Bonds In Cyclohexane Chemistry Libretexts.